|
|
|
| 114
|
Hui C.; Craggs L.; Antonchick, A.P. Ring
contraction in synthesis of functionalized carbocycles. Chem.
Soc. Rev., 2022, 51, 8652-8675, DOI: 10.1039/D1CS01080H
|

Carbocycles are a key and
widely present structural motif in organic compounds.
The construction of structurally intriguing
carbocycles, such as highly-strained fused rings,
spirocycles or highly-functionalized carbocycles
with congested stereocenters, remains challenging
in organic chemistry. Cyclopropanes, cyclobutanes
and cyclopentanes within such carbocycles can
be synthesized through ring contraction. These
ring contractions involve re-arrangement of
and/or small molecule extrusion from a parental
ring, which is either a carbocycle or a heterocycle
of larger size. This review provides an overview
of synthetic methods for ring contractions to
form cyclopropanes, cyclobutanes and cyclopentanes
en route to structurally intriguing carbocycles.
|
|
| 113
|
Hui C.; Antonchick, A.P. Methodology-driven
efficient synthesis of cytotoxic (±)-piperarborenine
B , Green Synth. Catal. 2022, advance article
DOI: 10.1016/j.gresc.2022.07.001
|
The evolution of synthetic
design toward the efficient synthesis of cyclobutane
natural product (±)-piperarborenine B
is demonstrated. Taking the advantages of good
functional group compatibility of contractive
synthesis of cyclobutanes from pyrrolidines,
stereoselective synthesis of unsymmetric highly
functionalized cyclobutanes core of (±)-piperarborenine
B was realized in one step. Also, an unprecedented
carboxylic acid assisted-diastereoselective
Kracho decarboxylation/transmethylation features
a new strategy for a non-symmetrical cyclobutane
core. The synthesis of (±)-piperarborenine
B illustrates the advancement of methodology
resulting in the improvement in synthetic efficiency.
|
|
| 112
|
M. Akbarzadeh, J. Flegel, S. Patil,
E. Shang, R. Narayan, M. Buchholzer, N. S. Kazemein Jasemi,
M. Grigalunas, A. Krzyzanowski, D. Abegg, A. Shuster, M.
Potowski, H. Karatas, G. Karageorgis, N. Mosaddeghzadeh,
M.-L. Zischinsky, C. Merten, C. Golz, L. Brieger, C. Strohmann,
A. P. Antonchick, P. Janning, A. Adibekian, R. S. Goody,
M. R. Ahmadian, S. Ziegler, H. Waldmann, The pseudo‐natural
product rhonin targets RHOGDI Angew. Chem. Int. Ed.
2022, 61, e202115193 DOI: 10.1002/anie.202115193;
Angew. Chem. 2022, 134, e202115193.
DOI: 10.1002/ange.202115193
|
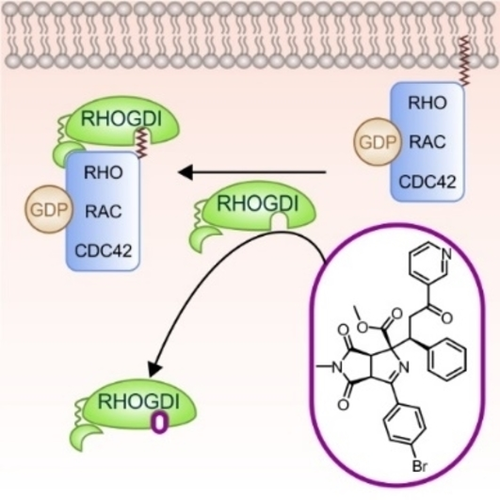
For the discovery of
novel chemical matter generally endowed with
bioactivity, strategies may be particularly
efficient that combine previous insight about
biological relevance, e.g., natural product
(NP) structure, with methods that enable efficient
coverage of chemical space, such as fragment-based
design. We describe the de novo combination
of different 5-membered NP-derived N-heteroatom
fragments to structurally unprecedented “pseudo-natural
products” in an efficient complexity-generating
and enantioselective one-pot synthesis sequence.
The pseudo-NPs inherit characteristic elements
of NP structure but occupy areas of chemical
space not covered by NP-derived chemotypes,
and may have novel biological targets. Investigation
of the pseudo-NPs in unbiased phenotypic assays
and target identification led to the discovery
of the first small-molecule ligand of the RHO
GDP-dissociation inhibitor 1 (RHOGDI1), termed
Rhonin. Rhonin inhibits the binding of the RHOGDI1
chaperone to GDP-bound RHO GTPases and alters
the subcellular localization of RHO GTPases.
|
|
| 111
|
Hui C.; Antonchick, A.P. Iodonitrene:
a direct metal-free electrophilic aminating reagent. Org.
Chem. Front., 2022, 9, 3897-3907, DOI: 10.1039/D2QO00739H
|

The use of conventional nitrenoids
and/or metal nitrenes as electrophilic aminating
reagents requires a pre-activated nitrogen atom,
which makes transfer of an unprotected NH-group
a difficult challenge. Iodonitrene, which is
generated in situ from phenyliodine(III) diacetate
and an ammonia surrogate, represents a new type
of reactive electrophilic aminating reagent.
The novel reactivity of iodonitrene not only
resulted in direct NH-group transfer to nucleophilic
atoms such as sulfur and nitrogen, but also
led to the development of new reactions such
as diazirine synthesis via decarboxylation and
contractive synthesis of cyclobutanes via nitrogen
extrusion. We highlight the contemporary advances
in the application of iodonitrene and discuss
the current limitations and future prospects.
|
|
| 110
|
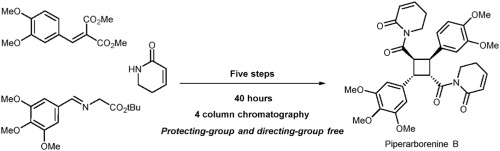
Hui C.; Antonchick, A.P. Concise synthesis
of piperarborenine B. Bioorg. Med. Chem. 2022,
67, 116817, DOI: 10.1016/j.bmc.2022.116817
Part of special issue: SI: Herbert Waldmann Honour Issue
|

A concise synthesis of piperarborenine
B is reported. Organocatalytic electrophilic
amination of pyrrolidines, stereospecific oxidative
ring contraction and an original diastereoselective
Krapcho dealkoxycarbonylation/transmethylation
contribute to a novel synthetic strategy to
the preparation of a non-symmetrical cyclobutane
core. Being transition-metal-free, directing-group-free
and protecting-group-free, a five-step synthesis
of piperarborenine B was accomplished.
|
|
| 109
|
Wesseler F.; Riege D.; Puthanveedu
M.; Halver J.; Müller E.; Bertrand J.; Antonchick A.P.;
Sievers S.; Waldmann H.; Schade D. Probing Embryonic Development
Enables the Discovery of Unique Small-Molecule Bone Morphogenetic
Protein Potentiators. J. Med. Chem. 2022,
65, 3978–3990, DOI: 10.1021/acs.jmedchem.1c01800
|
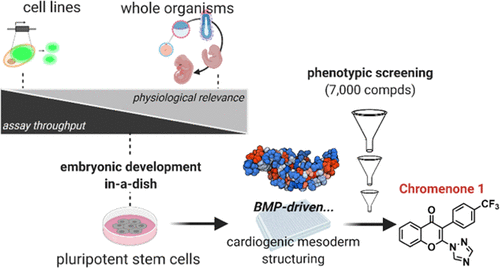
We report on the feasibility
to harness embryonic development in vitro for
the identification of small-molecule cytokine
mimetics and signaling activators. Here, a phenotypic,
target-agnostic, high-throughput assay is presented
that probes bone morphogenetic protein (BMP)
signaling during mesodermal patterning of embryonic
stem cells. The temporal discrimination of BMP-
and transforming growth factor-β (TGFβ)-driven
stages of cardiomyogenesis underpins a selective,
authentic orchestration of BMP cues that can
be recapitulated for the discovery of BMP activator
chemotypes. Proof of concept is shown from a
chemical screen of 7000 compounds, provides
a robust hit validation workflow, and afforded
2,3-disubstituted 4H-chromen-4-ones as potent
BMP potentiators with osteogenic efficacy. Mechanistic
studies suggest that Chromenone 1 enhances canonical
BMP outputs at the expense of TGFβ-Smads in
an unprecedented manner. Pharmacophoric features
were defined, providing a set of novel chemical
probes for various applications in (stem) cell
biology, regenerative medicine, and basic research
on the BMP pathway.
|
|
| 108
|
Puthanveedu M.; Antonchick, A.P. Aromatic
C–H functionalization. In Iodine Catalysis in Organic Synthesis
(eds K. Ishihara and K. Muñiz) (2022). DOI:
10.1002/9783527829569.ch6
|
Iodine catalysis has
enjoyed substantial progress in the area of
C–H functionalization chemistry. In this chapter,
we focus on the key developments of hypervalent
iodine-mediated transformations for the direct
C–H functionalization of aromatic and hetero-aromatic
compounds. Emphasis is given for novel transition-metal-free
methodologies using stoichiometric as well as
the catalytic amount of hypervalent iodine reagents.
|
|
| 107
|
Serebrennikova P.O.; Utepova I.A.;
Chupakhin O.N.; Guzhova I.V.; Mikhaylova E.R.; Antonchick
A.P. Synthesis and biological investigation of 1,2,4-triazolo[4,3-a]azines
as potential HSF1 inductors. Synthesis
2022; 54,
2677-2686, DOI: 10.1055/s-0040-1719907
|
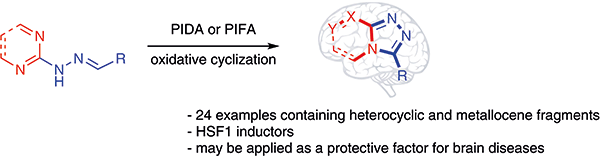
Derivatives of fused 1,2,4-triazines containing heterocyclic and
metallocene fragments were obtained by one-pot oxidative cyclization of
heterocyclic hydrazones in the presence of hypervalent iodine(III)
reagents. For 1,2,4-triazolo[4,3-a]azines, the
ability to activate HSF1 was investigated. The obtained compounds were
shown to increase the degree of HSF1 activation. It was shown that the
1,2,4-triazines can be used to induce Hsp70 expression and decrease the
extent of mutant HTT aggregate formation. |
|
| 106
|
Hui C.; Brieger L.; Strohmann, C.; Antonchick, A.P. Stereoselective Synthesis of Cyclobutanes by Contraction of Pyrrolidines. J. Am. Chem. Soc. 2021, 123, 18864-18870, DOI: 10.1021/jacs.1c10175
|

Here we report a contractive synthesis of multisubstituted cyclobutanes containing multiple stereocenters from readily accessible pyrrolidines using iodonitrene chemistry. Mediated by a nitrogen extrusion process, the stereospecific synthesis of cyclobutanes involves a radical pathway. Unprecedented unsymmetrical spirocyclobutanes were prepared successfully, and a concise, formal synthesis of the cytotoxic natural product piperarborenine B is reported.
|
|
| 105
|
Polychronidou, V.; Krupp, A.; Strohmann, C.; Antonchick, A.P. Cascade aza-Wittig/6 pi-Electrocyclization in the Synthesis of 1,6-Dihydropyridines. Org. Lett. 2021, 23, 6024-6029, DOI: 10.1021/acs.orglett.1c02099
|

A metal-free protocol for the synthesis of substituted 1,6-dihydropyridines with quaternary stereogenic centers via a cascade aza-Wittig/6 pi-electrocyclization process has been developed. The high functional group compatibility and broad scope of this method were demonstrated by using a wide range of easily available vinyliminophosphoranes and ketones, with yields up to 97%. A modification of the obtained products allowed for an increase in complexity and chemical diversity. Finally, attempts for asymmetric synthesis of 1,6-dihydropyridines are demonstrated.
|
|
| 104
|
Yildirim, O.; Grigalunas, M.; Brieger, L.; Strohmann, C.; Antonchick, A.P.*; Waldmann, H.* Dynamic Catalytic Highly Enantioselective 1,3-Dipolar Cycloadditions Angew. Chem., Int. Ed. 2021, 60, 20012-20020, DOI: 10.1002/anie.202108072
|

In dynamic covalent chemistry, reactions follow a thermodynamically controlled pathway through equilibria. Reversible covalent-bond formation and breaking in a dynamic process enables the interconversion of products formed under kinetic control to thermodynamically more stable isomers. Notably, enantioselective catalysis of dynamic transformations has not been reported and applied in complex molecule synthesis. We describe the discovery of dynamic covalent enantioselective metal-complex-catalyzed 1,3-dipolar cycloaddition reactions. We have developed a stereodivergent tandem synthesis of structurally and stereochemically complex molecules that generates eight stereocenters with high diastereo- and enantioselectivity through asymmetric reversible bond formation in a dynamic process in two consecutive Ag-catalyzed 1,3-dipolar cycloadditions of azomethine ylides with electron-poor olefins. Time-dependent reversible dynamic covalent-bond formation gives enantiodivergent and diastereodivergent access to structurally complex double cycloadducts with high selectivity from a common set of reagents.
|
|
| 103
|
Puthanveedu, M.; Khamraev, V.; Brieger, L.; Strohmann, C.; Antonchick, A.P. Electrochemical Dehydrogenative C(sp(2))-H Amination. Chem. Eur. J. 2021, 27, 8008-8012, DOI: 10.1002/chem.202100960
|

A transition-metal-free direct electrolytic C-H amination involving an electrochemically generated nitrenium ion intermediate has been developed. The electrosynthesis takes place in the absence of any organoiodine catalysts and is enabled by an in situ generated electrolyte. A novel, efficient intramolecular and intermolecular C-H amination has been demonstrated using a simple reaction setup.
|
|
| 102
|
Grigalunas, M.; Burhop, A.; Zinken, S.; Pahl, A.; Gally, J.M.; Wild, N.; Mantel, Y.; Sievers, S.; Foley, D.J.; Scheel, R.; Strohmann, C.; Antonchick, A.P.; Waldmann, H. Natural product fragment combination to performance-diverse pseudo-natural products. Nature Commun. 2021, 12, Article Number: 1883, DOI: 10.1038/s41467-021-22174-4
|

Natural product structure and fragment-based compound development inspire pseudo-natural product design through different combinations of a given natural product fragment set to compound classes expected to be chemically and biologically diverse. We describe the synthetic combination of the fragment-sized natural products quinine, quinidine, sinomenine, and griseofulvin with chromanone or indole-containing fragments to provide a 244-member pseudo-natural product collection. Cheminformatic analyses reveal that the resulting eight pseudo-natural product classes are chemically diverse and share both drug- and natural product-like properties. Unbiased biological evaluation by cell painting demonstrates that bioactivity of pseudo-natural products, guiding natural products, and fragments differ and that combination of different fragments dominates establishment of unique bioactivity. Identification of phenotypic fragment dominance enables design of compound classes with correctly predicted bioactivity. The results demonstrate that fusion of natural product fragments in different combinations and arrangements can provide chemically and biologically diverse pseudo-natural product classes for wider exploration of biologically relevant chemical space. Natural products inspire the development of pseudo-natural products through combinations of fragments of compound classes that are chemically and biologically distinct. Here, the authors report a library of 244 pseudo-natural products, evaluate them in the cell painting essays and identify the phenotypic role of individual fragments.
|
|
| 101
|
Shaaban, S.; Li, H.; Merten, C.; Antonchick, A.P.*; Waldmann, H.* Rhodium(III)-Catalyzed Enantioselective Benzamidation of Cyclopropenes. Synthesis 2021, 53, 2192-2200, DOI: 10.1055/s-0040-1706026
|

Cyclopropylamines are characteristic structural motifs found in a variety of natural products and pharmaceuticals and therefore engaging targets for the development of new methods for their synthesis. Herein the synthesis of enantioenriched cyclopropylamines through catalytic enantioselective C-H functionalization using a chiral RhJasCp complex is reported. The reaction proceeds under mild conditions with high enantiocontrol. This reaction enables access to cyclopropylamines with three contiguous stereocenters originating from the corresponding cyclopropenes.
|
|
| 100
|
Shaaban, S.; Li, H.; Otte, F.; Strohmann, C.; Antonchick, A.P.*; Waldmann, H.* Enantioselective Synthesis of Five-Membered-Ring Atropisomers with a Chiral Rh(III) Complex. Org. Lett. 2020, 22, 9199-9202, DOI: 10.1021/acs.orglett.0c03355
|

Axially chiral atropisomeric compounds are widely applied in asymmetric catalysis and medicinal chemistry, and efficient methods for their synthesis are in high demand. This applies in particular to atropisomers derived from five-membered aromatic rings because their lower barrier for rotation among the biaryl axis limits their asymmetric synthesis. We report here an enantioselective C-H functionalization method using our chiral RhJasCp complex for the synthesis of the biaryl atropisomer types that can be accessed from three different five-membered-ring heterocycles.
|
|
| 99
|
Puthanveedu M.; Polychronidou V., Antonchick A. P. Catalytic Selective Metal-Free Cross-Coupling of Heteroaromatic N-Oxides with Organosilanes Org. Lett., 2019, 21, 3407-3411, DOI: 10.1021/acs.orglett.9b01141
|

A metal-free, regioselective C–H functionalization of heteroaromatic N-oxides has been developed. The method enables the synthesis of various benzylated and alkynylated N-heterocycles in a transition-metal-free manner employing organosilanes as coupling partners. The unanticipated reactivity has been exploited for the synthesis of a number of symmetrical disubstituted acetylenes from ethynyltrimethylsilane via carbon–silicon bond metathesis.
|
|
| 98
|
Schneidewind T., Kapoor S., Garivet G., Karageorgis G., Narayan R., Vendrell-Navarro G., Antonchick A.P., Ziegler S., Waldmann H. The Pseudo Natural Product Myokinasib Is a Myosin Light Chain Kinase 1 Inhibitor with Unprecedented Chemotype Cell Chem. Biol., 2019, 26, P512-523.e5, DOI: 10.1016/j.chembiol.2018.11.014
|

Small-molecule chemotypes with unexpected bioactivity may be identified by combining strategies built on the biological relevance of, e.g., natural products (NPs), such as biology-oriented synthesis, with principles that enable efficient coverage of chemical space, such as fragment-based compound design. Evaluation in target-agnostic phenotypic assays and target identification may link biologically relevant chemotypes to unexpected and unknown targets. We describe the phenotypic identification of an unprecedented kinase inhibitor chemotype obtained by synthetic combination of two biosynthetically unrelated NP fragment types. Target identification and biological characterization revealed that the inhibitor, termed Myokinasib, impairs cytokinesis, induces formation of multinucleated cells, and reduces phosphorylated myosin II light chain abundance on stress fibers by selective inhibition of myosin light chain kinase 1.
|
|
| 97
|
Bering L., Antonchick A. P. Reactive nitrogen species: Nitrosonium ions in organic synthesis (Invited) Tetrahedron, 2019, 75, 1131-1143, DOI: 10.1016/j.tet.2019.01.036
|

Nitrosonium ions are versatile and mild oxidants, which were successfully employed in organic transformations. The applications cover different fields, such as the functionalization of carbon-carbon and carbon-heteroatom bonds, functionalization of unsaturated bonds and the oxidative coupling of arenes, catalyzed by nitrosonium ions. Due to the ability of nitrosonium ions to modify various types of bonds with different modes of action, a variety of applications has been established, which addresses current challenges in organic chemistry research. By considering additional points, such as safety of reagents, by-product formation, employed solvents and energy consumption, several aspects of green and sustainable chemistry can be addressed. Within this review, synthetic applications of nitrosonium ions under transition metal-free reaction conditions are summarized.
|
|
| 96
|
Bering L., D’Ottavio L., Sirvinskaite G., Antonchick A. P. Nitrosonium ion catalysis: aerobic, metal-free cross-dehydrogenative carbon–heteroatom bond formation Chem. Commun., 2018, 54, 13022-13025 , DOI: 10.1039/C8CC08328B
|

Catalytic cross-dehydrogenative coupling of heteroarenes with thiophenols and phenothiazines has been developed under mild and environmentally benign reaction conditions. For the first time, NOx+ was applied for catalytic C–S and C–N bond formation. A comprehensive scope for the C–H/S–H and C–H/N–H cross-dehydrogenative coupling was demonstrated with >60 examples. The sustainable cross-coupling conditions utilize ambient oxygen as the terminal oxidant, while water is the sole by-product.
|
|
| 95
|
Li H., Gontla R., Flegel J., Merten C., Ziegler S., Antonchick A. P.*, Waldmann H*. Enantioselective Formal C(sp3)‐H Bond Activation in the Synthesis of Bioactive Spiropyrazolone Derivatives. Angew. Chem., Int. Ed. 2019, 58, 307-311, DOI:10.1002/anie.201811041 Angew. Chem. 2019, 131, 313-317, DOI:10.1002/ange.201811041
|

Herein we report the first enantioselective annulation of α‐arylidene pyrazolones through a formal C(sp3)‐H activation under mild conditions enabled by highly variable Rh(III)‐Cpx catalysts. The method has wide substrate scope proceeds with good to excellent yields and enantioselectivity. Its synthetic utility was demonstrated by late‐stage functionalization of drugs and natural products as well as preparation of enantioenriched [3]‐dendralenes. Preliminary biological investigation also identified the spiropyrazolones as a novel class of Hedgehog pathway inhibitors.
|
|
| 94
|
Manna S., Antonchick A. P. Catalytic Transfer Hydrogenation Using Biomass as Hydrogen Source. ChemSusChem 2019, 12, 3094-3098, DOI: 10.1002/cssc.201801770
|

Sweet chemistry. A catalytic transfer hydrogenation using biomass‐derived carbohydrates as reagent is developed. Stereoselective and chemoselective hydrogenation of alkynes, alkenes, and carbonyl compounds is demonstrated. This work provides an operationally simple method for transfer hydrogenation and represents a new concept for the application of renewable biomass.
|
|
| 93
|
Shan G., Flegel J., Li H., Merten C., Ziegler S., Antonchick A. P.*, Waldmann H*. C–H Bond Activation for the Synthesis of Heterocyclic Atropisomers Yields Hedgehog Pathway Inhibitors. Angew. Chem., Int. Ed. 2018, 57, 14250-14254, DOI:10.1002/anie.201809680, Angew. Chem. 2018, 130, 14446-14450, DOI:10.1002/ange.201812882
|

Axially chiral 4‐arylisoquinolones are endowed with pronounced bioactivity, and methods for their efficient synthesis have gained widespread attention. However, enantioselective synthesis of axially chiral 4‐arylisoquinolones by means of C‐H activation has not been reported to date. We describe rhodium (III)‐catalyzed C‐H bond activation and annulation for the atroposelective synthesis of axially chiral 4‐arylisoquinolones. The method employs chiral cyclopentadienyl ligands embodying a piperidine ring as backbone and yields the atropisomers with good to excellent yields and enantioselectivity. Biological relevance of the 4‐arylisoquinolones was demonstrated by their investigation in different cellular assays, leading to the discovery of novel non‐SMO binding Hedgehog pathway inhibitors.
|
|
| 92
|
Utepova I.A., Serebrennikova P.O., Streltsova M.S., Musikhina A. A., Fedorchenko T.G., Chupakhin O.N. Antonchick A.P. Enantiomerically Enriched 1,2-P,N-Bidentate Ferrocenyl Ligands for 1,3-Dipolar Cycloaddition and Transfer Hydrogenation Reactions. Molecules 2018, 23, 1311. DOI:10.3390/molecules23061311
|

Novel complexes of 1,2-P,N-bidentate ferrocenyl ligands with AgOAc or with [RuCl2(PPh3)3] as catalysts have been studied in asymmetric synthesis. The catalytic activity of these systems have been studied in [3+2]-cycloaddition of azomethine ylides with olefins and the asymmetric transfer hydrogenation of ketones.
|
|
| 91
|
Bering L., Antonchick A.P. Oxidative Heteroatom–Heteroatom Bond Formation' in Patai’s Chemistry of Functional Groups, edited by Ilan Marek, Berit Olofsson, Zvi Rappoport. John Wiley & Sons, Ltd: Chichester, UK, 2018. DOI: 10.1002/9780470682531.pat0946
|
The development of oxidative heteroatom–heteroatom bond‐forming reactions based on the use of hypervalent iodine reagents is summarized, with emphasis on metal‐free and transition‐metal‐catalyzed reactions. The most important approaches are located in the field of nitrogen–heteroatom and sulfur–heteroatom bond formation, but also chemical transformations including rather rarely used elements are discussed. Increasing interest in the field of heteroatom–heteroatom bond formation in the past decades led to some notable discoveries, which are discussed within this chapter.
|
|
| 90
|
Bering L., Vogt M., Paulussen F.M., Antonchick A.P. Selective, Catalytic, and Metal-Free Coupling of Electron-Rich Phenols and Anilides Using Molecular Oxygen as Terminal Oxidant Org. Lett., 2018, 20, 4077-4080 DOI:10.1021/acs.orglett.8b01631
| 
Selective oxidative homo- and cross-coupling of electron-rich phenols and anilides was developed using nitrosonium tetrafluoroborate as a catalyst. Oxidative coupling of phenols revealed unusual selectivities, which translated into the unprecedented synthesis of inverse Pummerer-type ketones. Mechanistic studies suggest that oxidative coupling of phenols and anilides shares a common pathway via homolytical heteroatom–hydrogen bond cleavage. Nitrosonium salt catalysis was applied for cross-dehydrogenative coupling initiated by generation of heteroatom-centered radicals.
|
|
| 89
|
Bering L., Jeyakumar K., Antonchick A.P. Metal-Free C–O Bond Functionalization: Catalytic Intramolecular and Intermolecular Benzylation of Arenes. Org. Lett., 2018, 20, 3911-3914 DOI:10.1021/acs.orglett.8b01495
| 
A catalytic, metal-free intramolecular rearrangement of benzyl phenyl ethers using nitrosonium salt as a catalyst is described. The optimized reaction conditions enabled a catalytic and metal-free Friedel–Crafts alkylation reaction with benzylic alcohols, producing water as the stoichiometric byproduct. A comprehensive scope (>50 examples) for both approaches and application in drug synthesis were demonstrated. Mechanistic studies suggest a Lewis acid-based mechanism for the metal-free Friedel–Crafts reaction.
|
|
| 88
|
Bering L., Paulussen F.M., Antonchick A.P. Aerobic, Metal-Free, and Catalytic Dehydrogenative Coupling of Heterocycles: En Route to Hedgehog Signaling Pathway Inhibitors. Org. Lett., 2018, 20, 1978-1981 DOI:10.1021/acs.orglett.8b00521
| 
The nitrosonium ion-catalyzed dehydrogenative coupling of heteroarenes
under mild reaction conditions is reported. The developed method
utilizes ambient molecular oxygen as a terminal oxidant, and only water
is produced as byproduct. Dehydrogenative coupling of heteroarenes
translated into the rapid discovery of novel hedgehog signaling pathway
inhibitors, emphasizing the importance of the developed methodology.
|
|
| 87
|
Murarka S.*, Antonchick A. P.* Metal-Catalyzed Oxidative Coupling of Ketones and Ketone Enolates (invited minireview) Synthesis 2018, 50, 2150-2162 DOI: 10.1055/s-0037-1609715
| 
Recent years have witnessed a significant advancement in the field of
radical oxidative coupling of ketones towards the synthesis of highly
useful synthetic building blocks, such as 1,4-dicarbonyl compounds, and
biologically important heterocyclic and carbocyclic compounds. Besides
oxidative homo- and cross-coupling of enolates, other powerful methods
involving direct C(sp3)–H functionalizations of ketones have
emerged towards the synthesis of 1,4-dicarbonyl compounds. Moreover,
direct α-C–H functionalization of ketones has also allowed an efficient
access to carbocycles and heterocycles. This review summarizes all these
developments made since 2008 in the field of metal-catalyzed/promoted
radical-mediated functionalization of ketones at the α-position.
|
|
| 86
|
Matcha K., Antonchick A.P. Transition Metal‐Free Radical Hydrotrifluoromethylation of Alkynes (invited article for special issue: Organic Reaction Mechanisms) Eur. J. Org. Chem. 2019, 309-312, DOI:10.1002/ejoc.201800291
| 
A combination of readily available and bench stable CF3SO2Na and t-BuOOH was efficiently
used for hydrotrifluoromethylation of alkynes. An excellent trans-selectivity was
demonstrated in the synthesis of alkenes. The developed mild reaction conditions allow
to suppress the competing Meyer-Schuster type rearrangement.
|
|
| 85
|
Jia Z-J., Shan G., Daniliuc C.G., Daniliuc C.G., Antonchick A. P.*, Waldmann H*. Enantioselective Synthesis of the Spirotropanyl Oxindole Scaffold through Bimetallic Relay Catalysis. Angew. Chem., Int. Ed. 2018, 57, 14493-14497, DOI:10.1002/anie.201712882. Angew. Chem. 2018, 130, 14701-14705, DOI:10.1002/ange.201712882
| 
Spirotropanyl oxindole alkaloids like alstonisine and chitosenine show a wide range
of bioactivites. We report the first enantioselective synthesis of the spirotropanyl
oxindole scaffold by means of a bimetallic relay catalysis strategy. A new class of
E-oximino a-diazo ketones was developed for the intramolecular generation of transient
azomethine ylides catalyzed by an achiral RhII complex and a subsequent intermolecular 1,3‐dipolar cycloaddition catalyzed by a
chiral N,N'-dioxide NdIII Lewis acid complex. The enantioselectively catalyzed transformation has broad scope
and yields the desired spirotropanyl oxindole cycloadducts in high yields and with
very high enantio‐ and diastereoselectivity.
|
|
| 84
|
Jia Z-J., Takayama H., Futamura Y., Aono H., Bauer J.O., Strohmann C., Antonchick A.P.*, Osada H., Waldmann H.* Catalytic Enantioselective Synthesis of a Pyrrolizidine–Alkaloid-Inspired Compound Collection with Antiplasmodial Activity.(invited article) J. Org. Chem., 2018, 83, 7033-7041, DOI: 10.1021/acs.joc.7b03202
| 
A novel enantioselective approach to the synthesis of a compound collection inspired by natural pyrrolizidine alkaloids was developed, employing an enantioselectively catalyzed 1,3-dipolar cycloaddition as the key step. The cycloadducts were obtained with excellent enantio- and diastereoselectivity. Biological evaluation of the resulting compound collection revealed that the compound class has multiple bioactivities, including activity against Plasmodium falciparum 3D7 and inhibition of Hedgehog signaling.
|
|
| 83
|
Joseph J., Antonchick A. P. Free Radicals in Heterocycle Functionalization. Topics in Heterocyclic Chemistry, 2017, 54, 93-149 DOI: 10.1007/7081_2017_8|
|
Functionalization of heterocycles through free radical intermediates has been widely employed in a diverse array of synthetic transformations. This chapter focuses on the recent developments in the light-assisted as well as traditional free radical generation methodology and the subsequent utilization in functionalization of various heterocycles.
|
|
| 82
|
Xu H., Laraia L., Schneider L., Louven K., Strohmann C., Antonchick A. P.*, Waldmann H*. Highly Enantioselective Catalytic Vinylogous Propargylation of Coumarins Yields a Class of Autophagy Inhibitors. Angew. Chem., Int. Ed. 2017, 56, 11232-11236 , DOI:10.1002/anie.201706005. Angew. Chem. 2017, 129, 11384-11388, DOI:10.1002/ange.201706005
| 
Class act: In the copper-catalyzed title reaction, aromatic and aliphatic propargylic esters react with substituted coumarins to give the desired products in excellent yields and enantioselectivities. Single-step transformations enabled the synthesis of several compounds. Biological investigation of the compound collection led to the discovery of a novel class of autophagy inhibitors.
|
|
| 81
|
Bering L., Manna S., Antonchick A. P. Sustainable Oxidative Metal-Free Annulation. Chem. Eur. J. 2017, 23, 10936-10946, DOI:10.1002/chem.201702063
| 
The combination of annulation strategies and direct C–H bond functionalization reactions allow the efficient synthesis of cyclic molecules using readily available and non-prefunctionalized precursors. Metal-free oxidative coupling via C–H bond functionalization is a green and sustainable approach due to its environmental benign and step- and atom-economic advantages. This concept highlights novel strategies and recent breakthroughs for metal-free
annulation via C–H bond functionalization giving access to a variety of important structural motifs.
|
|
| 80
|
Manna S., Antonchick A. P. Metal-Free Oxidative Dehydrogenative Diels-Alder Reaction for Selective Functionalization of Alkylbenzenes. Chem. Eur. J. 2017, 23, 7825-7829, DOI:10.1002/chem.201701535
| 
Functionalization of C(sp3)-H bonds under metal-free reaction conditions is a great challenge due to low bond reactivity. A novel metal-free oxidative dehydrogenative Diels-Alder reaction of alkylbenzene derivatives with alkenes via C(sp3)-H bond functionalization is described. The developed oxidative method provides a straightforward approach to biologically relevant 1,4-phenanthraquinone and isoindole derivatives from readily available starting
materials. Furthermore, the synthesis of nitrostyrenes from enylbenzene derivatives via selective C(sp3)-H bond functionalization has been demonstrated.
|
|
| 79
|
Förster T., Lopéz-Tosco S., Ziegler S., Antonchick A. P., Waldmann H. Enantioselective Organocatalytic Synthesis of a Secoyohimbane-Inspired Compound Collection with Neuritogenic Activity. ChemBioChem 2017, 56, 1098-1108, DOI:10.1002/cbic.201700015.
| 
Natural products provide evolutionary validated core structures inspiring the synthesis of new compound collections endowed with neurite growth-promoting activity. Rhynchophylline is the major component of Uncaria species which has been used to treat neurological diseases in Chinese traditional medicine. According to the structure of this spirocyclic secoyohimbane alkaloid, we developed a highly enantioselective and efficient organocatalyzed synthesis
method to provide the tetracyclic secoyohimbane scaffold incorporating a quaternary and three tertiary stereogenic centers in a one-pot multistep reaction sequence. A compound collection of derived secoyohimbanes was synthesized and expanded decorating the periphery of the basic scaffold with additional substituents to increase the diversity and the number of the library members. Evaluation of the different sub-collections of secoyohimbanes for modulation of neurite outgrowth in the SH-SY5Y human cell line led
to the discovery of new compounds that promote neurite outgrowth.
|
|
| 78
|
Jia Z-J., Merten C., Gontla R., Daniliuc C.G., Antonchick A. P.*, Waldmann H*. General Enantioselective C−H Activation with Efficiently Tunable Cyclopentadienyl Ligands. Angew. Chem., Int. Ed. 2017, 56, 2429-2434, DOI:10.1002/anie.201611981. Angew. Chem. 2017, 129, 2469-2474, DOI:10.1002/ange.2016011981
| 
The discovery of chiral Cp ligands that unite the advantages of previously developed ligand classes is enabled by a novel approach. They can be readily synthesized on gram scale, and both their structures and configurations can be efficiently adjusted by means of flexible enantioselective [6+3] cycloaddition reactions. With these ligands, three rhodium(III)-catalyzed C−H activation reactions were rendered highly enantioselective.
|
|
| 77
|
Bering L., Antonchick A. P. Selective Transition-Metal-Free Vicinal cis-Dihydroxylation of Saturated Hydrocarbons. Chem. Sci. 2017, 8, 452-457, DOI:10.1039/C6SC03055F.
| 
Transition-metal-free cis-dihydroxylation of saturated hydrocarbons under ambient reaction conditions has been developed. The described approach allows direct and selective synthesis of vicinal diols. The new reaction proceeds thereby via radical iodination and a sequence of oxidation steps. A broad scope of one-pot dual C(sp3)-H bond functionalization for the selective synthesis of vicinal syn-diols
was demonstrated.
|
|
| 76
|
Murarka S., Golz C., Strohmann C., Antonchick A. P.*, Waldmann H*. Biology-Oriented Synthesis of 3,3-Spiro(2-tetrahydrofuranyl)oxindoles. Synthesis 2017, 49, 87-95, DOI:10.1055/s-0035-1561665. (Dedicated to Prof. Dieter Enders on the occasion of his 70th birthday)
| 
A biology-oriented synthesis of 3,3-spiro(2-tetrahydrofuranyl)oxindole derivatives is realized through the rhodium(II)-catalyzed three-component reaction of diazoamides, aldehydes and β-nitrostyrenes. The reactions are conducted under mild conditions and the products are obtained in moderate to good yields with excellent regio- and diastereoselectivity.
|
|
| 75
|
Caporaso R., Manna S., Zinken S., Kochnev A.R.,Lukyanenko E.R., Kurkin A.V., Antonchick A. P. Radical Trideuteromethylation with Deuterated Dimethyl Sulfoxide in the Synthesis of Heterocycles and Labelled Building Blocks. Chem. Commun., 2016, 12486-12489, DOI:10.1039/C6CC07196A .
| 
The potential of deuterated pharmaceuticals is being widely demonstrated. Here we describe the first trideuteromethylation under radical reaction conditions using deuterated dimethyl sulfoxide as reagent for the synthesis of labelled heterocycles and trideuteromethylated compounds. A broad scope of developed method for the synthesis of various scaffolds was demonstrated.
|
|
| 74
|
Xu H., Golz C., Strohmann C., Antonchick A. P.*, Waldmann H*. Enantiodivergent Combination of Natural Product Scaffolds Enabled by Catalytic Enantioselective Cycloaddition. Angew. Chem., Int. Ed. 2016, 55, 7761-7765, DOI:10.1002/anie.201602084. Angew. Chem. 2016, 128, 7892-7896, DOI:10.1002/ange.201602084
| 
Two for one offer: An efficient method based on copper(I)-catalyzed [3+2] cycloadditions has been developed for the enantiodivergent combination of natural product derived tropane and pyrrolidine scaffolds. The strategy enables the synthesis of two enantiopure products in a one-pot reaction with one chiral catalyst in high diastereo- and enantioselectivity.
|
|
| 73
|
Song Z., Antonchick A.P. Iridium(III)-catalyzed regioselective C7-sulfonamidation of indoles. Org. Biomol. Chem., 2016, 14, 4804-4808, DOI: 10.1039/C6OB00926C
| 
Iridium(III)-catalyzed direct C7-sulfonamidation of indoles with sulfonyl azides is described. The developed method has good compatibility with diverse functional groups, providing various 7-amino-substituted indoles with good to excellent yields in a short time under mild reaction conditions. The key feature of the developed method is the regioselective functionalization at the C7-position of 2,3-unsubstituted indoles. Biologically active compounds
can be obtained using this protocol. The application of the iridium(III) catalyst and directing group plays a crucial role in the regioselectivity of the developed reaction.
|
|
| 72
|
Song Z., Antonchick A.P. Catching α-aminoalkyl radicals: cyclization between tertiary alkylanilines and alkenes. Tetrahedron, 2016, 72, 7715-7721, DOI:10.1016/j.tet.2016.04.052
Tetrahedron Symposium in Print Radical Chemistry
| 
A practical synthesis of polycyclic heterocycles by radical annulation of tertiary arylamine derivatives is described. Radical annulation occurs via formation of α-aminoalkyl radicals. The reaction is initiated by tetrabutylammonium iodide using tert-butyl hydroperoxide as oxidant. The developed method allows functionalization of diverse tertiary alkylanilines with different alkenes.
|
|
| 71
|
Manna S., Antonchick A. P. [1+1+1] Cyclotrimerization for the Synthesis of Cyclopropanes. Angew. Chem., Int. Ed. 2016, 55, 5290-5293, DOI:10.1002/anie.201600807. Angew. Chem. 2016, 128, 5376-5379, DOI:10.1002/ange.201600807
Angewandte VIP (Open Access). One of the most read articles in March 2016. Highlighted in Chemical & Engineering News 2016, vol. 94, is. 16, p. 10. Science & Technology Concentrates and Angew. Chem., Int. Ed. 2016, 55, DOI:10.1002/anie.201602891 by John C. Walton: A Valuable Upgrade to the Portfolio of Cycloaddition Reactions. ChemInform
Editor’s Choice (RxnFinder)
| 
The synthesis of small rings by functionalization of C(sp3)−H bonds remains a great challenge. We report for the first time a copper-catalyzed [1+1+1] cyclotrimerization of acetophenone derivatives under mild reaction conditions. The reaction has a broad scope for the stereoselective synthesis of cyclopropanes by trimerization of acetophenone. The developed transformation is based on an extraordinary copper-catalyzed cascade process
that allows saturated carbocycles to be obtained for the first time by cyclotrimerization through functionalization of C(sp3)−H bonds. The cascade of sixfold C(sp3)−H bond functionalization allows the synthesis of cyclopropanes in a highly stereoselective approach.
|
|
| 70
|
Sellstedt M., Schwalfenberg M., Ziegler S., Antonchick A.P., Waldmann H. Trienamine catalyzed asymmetric synthesis and biological investigation of a cytochalasin B-inspired compound collection. Org. Biomol. Chem., 2016, 14, 50-54, DOI: 10.1039/C5OB02272J
| 
Due to their enhanced metabolic needs many cancers need a sufficient supply of glucose, and novel inhibitors of glucose import are in high demand. Cytochalasin B (CB) is a potent natural glucose import inhibitor which also impairs the actin cytoskeleton leading to undesired toxicity. With a view to identifying selective glucose import inhibitors we have developed an enantioselective trienamine catalyzed synthesis of a CB-inspired compound collection.
Biological analysis revealed that indeed actin impairment can be distinguished from glucose import inhibition and led to the identification of the first selective glucose import inhibitor based on the basic structural architecture of cytochalasin B.
|
|
| 69
|
Manna S., Serebrennikova P.O., Utepova I.A., Antonchick A.P.*, Chupakhin O.N.*. Hypervalent Iodine(III) in Direct Oxidative Amination of Arenes with Heteroaromatic Amines. Org. Lett., 2015, 17, 4588-4591, DOI:10.1021/acs.orglett.5b02320
One of the most read articles in September 2015 and listed in the top 20 most downloaded articles for the previous 12 months.
| 
A novel, mild, and practical method of amination of simple nonfunctionalized arenes under metal free conditions has been developed. The approach allows coupling of electron-rich arenes with amino derivatives of electron-deficient heterocycles providing rapid access to scaffolds of bioactive compounds and is based on the application of the hypervalent iodine(III) reagent as an oxidant. Regioselective functionalization of C–H bonds of arenes by the
formation of C–N bonds under organocatalytic conditions was demonstrated.
|
|
| 68
|
Manna S., Antonchick A. P. Copper(I)-Catalyzed Radical Addition of Acetophenones to Alkynes in Furan Synthesis. Org. Lett., 2015, 17, 4300-4303, DOI:10.1021/acs.orglett.5b02114
One of the most read articles in August and September 2015 and listed in the top 20 most downloaded articles for the previous 12 months.
| 
A synthesis of multisubstituted furans from readily available acetophenones and electron-deficient alkynes via direct C(sp3)−H bond functionalization under radical reaction conditions is described. The developed transformation is catalyzed by copper(I) salts using di-tert-butyl peroxide as an external oxidant. This method offers an efficient access to biologically important scaffolds from simple compounds.
|
|
| 67
|
Narayan R., Matcha K., Antonchick A. P. Metal-Free Oxidative C-C Bond Formation through C-H Bond Functionalization. Chem. Eur. J., 2015, 21, 14678-14693, DOI:10.1002/chem.201502005
The most read article from ChemPubSoc Europe journals
in October 2015 and one of the most accessed articles in first year after publication.
| 
Who needs metals anyway? This Minireview covers the rapidly growing area of metal-free oxidative C-C bond formation through direct C-H bond functionalization. A selection of recent important developments in this area is presented along with discussions of their reaction mechanisms.
|
|
| 66
|
Murarka S., Antonchick A. P. Oxidative Heterocycle Formation Using Hypervalent Iodine(III) Reagents. Top. curr. chem. 2016, 373, 75-104, DOI:10.1007/128_2015_647
|
Hypervalent iodine(III) reagents have been widely exploited in a diverse array of synthetic transformations. This chapter focuses on the general application of hypervalent iodine(III) reagents in the de novo synthesis and in the late stage functionalization of heterocyclic compounds.
|
|
| 65
|
Bering L., Antonchick A. P. Regioselective Metal-Free Cross-Coupling of Quinoline N-Oxides with Boronic Acids. Org. Lett., 2015, 17, 3134-3137, DOI:10.1021/acs.orglett.5b01456
| 
A novel and operationally simple one-step C–H bond functionalization of quinoline N-oxides to 2-substituted quinolines was developed. This approach enables the regioselective functionalization under external oxidant- and metal-free conditions. The developed transformation represents the first application of the Petasis reaction in functionalization of heterocycles.
|
|
| 64
|
Manna S., Antonchick A. P. Copper-Catalyzed (2+1) Annulation of Acetophenones with Maleimides: Direct Synthesis of Cyclopropanes. Angew. Chem., Int. Ed. 2015, 54, 14845-14848, DOI:10.1002/anie.201502872. Angew. Chem. 2015, 127, 15058-15061, DOI:10.1002/ange.201502872
| 
A practical synthesis of azabicyclo[3.1.0]hexane derivatives by direct oxidative coupling of arylmethyl ketones and maleimides was developed. The dehydrogenative annulation involves a double C-H bond functionalization at the α-position of the ketone using a copper(II) complex as the catalyst and di-tert-butyl peroxide as the oxidant.
|
|
| 63
|
Narayan R., Manna S., Antonchick A. P. Hypervalent Iodine(III) in Direct Carbon–Hydrogen Bond Functionalization. (Invited account) Synlett 2015, 26, 1785-1803, DOI:10.1055/s-0034-1379912
| 
Direct methods of novel bond formation through functionalization of nonreactive carbon–hydrogen bonds represent an efficient synthetic approach. Cross-dehydrogenative coupling has emerged as an area of huge potential and importance for novel bond formation. The application of hypervalent iodine(III) reagents in direct carbon–hydrogen bond functionalization reactions is of immense interest because the functionalization of the nonreactive carbon–hydrogen
bonds proceeds under metal-free reaction conditions. This account covers recent developments in the area of hypervalent iodine(III) mediated direct carbon–hydrogen bond functionalization.
|
|
| 62
|
Murarka S., Jia Z.-J., Merten C., Daniliuc C.-G., Antonchick A. P.*, Waldmann H*. Rhodium(II)-Catalyzed Enantioselective Synthesis of Troponoids. Angew. Chem., Int. Ed. 2015, 54, 7653-7656, DOI:10.1002/anie.201502233. Angew. Chem. 2015, 127, 7763-7766, DOI:10.1002/ange.201502233
Highlighted in Nachrichten aus der Chemie 2015, 63, 875 and Synfacts 2015, 0824.
| 
Decisive dipoles: In the rhodium(II)-catalyzed asymmetric 1,3-dipolar cycloaddition of tropone with carbonyl ylides, a programmable chemoselective reaction with the keto group or the 6 π system of tropone was controlled by the substrate (see scheme). The developed method enables the synthesis of complex products in highly enantiomerically enriched form.
|
|
| 61
|
Potowski M., Golz C., Strohmann C., Antonchick A. P.*, Waldmann H*. Biology-oriented synthesis of benzopyrano[3,4-c]pyrrolidines. (SIP Maja Köhn) Bioorg. Med. Chem., 2015, 23, 2895-2903, DOI: 10.1016/j.bmc.2015.02.044
| 
A natural product inspired synthesis of 6,6,5-tricyclic compounds via a silver(I)-catalyzed formal 1,3-dipolar cycloaddition of coumarins with α-iminoesters was developed. The reaction proceeds in a stepwise reaction course under formation of the trans-substituted diastereomer with respect to the 1,3-dipole and shows a broad substrate scope.
|
|
| 60
|
Manna S., Narayan R., Golz C., Strohmann C., Antonchick A. P. Regioselective Annulation of Nitrosopyridine with Alkynes: Straightforward Synthesis of N-Oxide-imidazopyridines. Chem. Commun., 2015, 51, 6119-6122, DOI: 10.1039/c5cc00533g
| 
We have developed a novel method for the regioselective annulation of 2-nitrosopyridines with variably substituted alkynes under mild reaction conditions. This approach allows the annulation of alkynes with 2-nitrosopyridines under reagent- and catalyst-free reaction conditions. The developed method shows excellent functional group tolerance and provides easy access to N-oxide-imidazo[1,2-a]pyridines.
|
|
| 59
|
Potowski M., Merten C., Antonchick A. P.*, Waldmann H* Catalytic Aerobic Oxidation and Tandem Enantioselective Cycloaddition in Cascade Multicomponent Synthesis. Chem. Eur. J. 2015, 21, 4913-4917, DOI: 10.1002/chem.201500125
| 
A new cascade reaction for the synthesis of complex products with eight stereocenters was developed. The reaction cascade proceeds with excellent diastereo- and enantioselectivity from simple starting chemicals such as aromatic aldehydes, amino acid esters, cyclopentadiene and molecular oxygen.
|
|
| 58
|
Samanta R., Narayan R., Bauer J. O., Strohmann C., Sievers S., Antonchick A. P. Oxidative Regioselective Amination of Chromones Exposes Potent Inhibitors of Hedgehog Signaling Pathway. Chem. Commun., 2015, 51, 925-928, DOI: 10.1039/C4CC08376H
| 
An operationally simple, transition metal-free, oxidative, regioselective cross-coupling between non-functionalized azoles and chromones was developed using iodine. The C2- position of chromone can be selectively coupled with nitrogen containing heterocycles. A broad scope of reaction with respect to coupling partners and further transformation of obtained products was demonstrated. The biological evaluation of obtained products revealed a novel
class of hedgehog signaling pathway inhibitors.
|
|
| 57
|
Jia Z.-H., Daniliuc C.G., Antonchick A.P.*, Waldmann H.* Phosphine-catalyzed dearomatizing [3+2] annulations of isoquinolinium methylides with allenes. Chem. Commun., 2015, 51, 1054-1057, DOI: 10.1039/C4CC08555H
| 
A phosphine-catalyzed dearomatizing [3+2] annulation of isoquinolinium methylides with allenoates or allenones yields highly functionalized pyrroloisoquinolines with high regioselectivity and in viable yields.
|
|
| 56
|
Matcha K., Antonchick A.P. Cascade Multicomponent Synthesis of Indoles, Pyrazoles, and Pyridazinones by Functionalization of Alkenes. Angew. Chem., Int. Ed. 2014, 53, 11960-11964, DOI:10.1002/anie.201406464. Angew. Chem. 2014, 126, 12154-12158, DOI:10.1002/ange.20146464
Highlighted in Synfacts 2015, 21
| 
Fischer-man’s friend: A regioselective multicomponent route to indole synthesis by functionalization of simple alkenes has been developed and has a broad scope. The novel application of alkene trifluoromethylation provides entry into Fischer indole synthesis, and various trifluoromethylated nitrogen heterocycles can be conveniently obtained.
|
|
| 55
|
Manna S., Matcha K., Antonchick A.P. Metal-Free Annulation of Arenes with 2-Aminopyridine Derivatives: The Methyl Group as a Traceless Non-Chelating Directing Group. Angew. Chem., Int. Ed. 2014, 53, 8163-8166, DOI:10.1002/anie.201403712. Angew. Chem. 2014, 126, 8302-8305, DOI:10.1002/ange.201403712
| 
Disappearing Me: A novel selective annulation between 2-aminopyridine derivatives and arenes under metal-free conditions provides the important pyrido[1,2-a]benzimidazole scaffold under mild reaction conditions. In this intermolecular reaction the methyl group of methylbenzenes serves as a traceless, non-chelating, and highly regioselective directing group.
|
|
| 54
|
Manna S., Antonchick A.P. Organocatalytic Oxidative Annulation of Benzamide Derivatives with Alkynes. Angew. Chem., Int. Ed. 2014, 53, 7324-7327, DOI:10.1002/anie.201404222. Angew. Chem. 2014, 126, 7452-7455, DOI:10.1002/ange.201404222
The most read article of ChemPubSoc Europe journals
in May 2014. Highlighted in Synfacts 2014, 871 and ChemCatChem DOI:10.1002/cctc.201402704
| 
Annulation without hesitation: N-alkoxybenzamides convert into isoquinolones smoothly and rapidly under organocatalytic conditions. The annulation of unsymmetrical diarylacetylenes proceeds with a high regioselectivity. The transformation is based on the hypervalent-iodine mediated generation of nitrenium ions.
|
|
| 53
|
Narayan R., Potowski M., Jia Z-J., Antonchick A.P.*, Waldmann H.* Catalytic Enantioselective 1,3-Dipolar Cycloadditions of Azomethine Ylides for Biology-Oriented Synthesis. Acc. Chem. Res., 2014, 47, 1296-1310, DOI: 10.1021/ar400286b
ACS Editors' Choice, One of the most read article in 4/2014-5/2014 and one of the most read article in 12 months.
| 
This Account highlights examples, mostly from our work, of the application of 1,3-dipolar cycloaddition reactions of azomethine ylides for the catalytic enantioselective synthesis of complex products. We successfully applied the 1,3-dipolar cycloaddition in the synthesis of spiro-compounds such as spirooxindoles, for kinetic resolution of racemic compounds in the synthesis of an iridoid inspired compound collection and in the synthesis of a nitrogen-bridged
bicyclic tropane scaffold by application of 1,3-fused azomethine ylides. Furthermore, we performed the synthesis of complex molecules with eight stereocenters using tandem cycloadditions. In a programmable sequential double cycloaddition, we demonstrated the synthesis of both enantiomers of complex products by simple changes in the order of addition of chemicals. Complex products were obtained using enantioselective higher order [6 + 3] cycloaddition of azomethine ylides with fulvenes followed by Diels–Alder
reaction. The bioactivity of these compound collections is also discussed.
|
|
| 52
|
Narayan R., Antonchick A.P. Hypervalent Iodine-Mediated Selective Oxidative Functionalization of (Thio)chromones with Alkanes. Chem. Eur. J. 2014, 20, 4568-4572, DOI: 10.1002/chem.201400186
One of the most read articles in 3/2014 and one of the most accessed articles in first year after publication.
| 
C-C bond formation is the most fundamental way for the chain propagation in organic molecules. This achievement through tandem oxidation of two different C-H bonds represents the state of the art in organic synthesis. Selective functionalization of the ubiquitous aliphatic C-H bonds offers an attractive option for this oxidative cross-coupling methodology. To develop such a methodology under mild and “metal-free” conditions remains challenging.
Herein, we report hypervalent iodine-mediated selective oxidative functionalization of aliphatic C-H bonds of alkanes with chromones and (thio)chromones. A wide range of alkanes, both cyclic and acyclic, has been found to react selectively and predictably in good yields. The developed methodology is also the first report of a direct oxidative functionalization of the C-2 position of (thio)chromones with alkanes to access bioactive compounds.
|
|
| 51
|
Narayan R., Antonchick A.P. 2,2'-Diiodobiphenyl. e-EROS. Encyclopedia of Reagents for Organic Synthesis (2014) DOI: 10.1002/047084289X.rn01648
|
| 50
|
Bruss H., Schuster H., Martinez R., Kaiser M., Antonchick A.P., Waldmann H. Synthesis of the B-seco limonoid core scaffold. Beilstein J. Org. Chem. 2014, 10, 194-208, DOI: 10.3762/bjoc.10.15
| 
Synthetic investigations towards the structurally complex and highly decorated framework of B-seco limonoid natural products by means of a [3,3]-sigmatropic rearrangement are described. Detailed model studies reveal, that an Ireland–Claisen rearrangement can be employed to construct the central C9–C10 bond thereby giving access to the B-seco limonoid scaffold. However, application of the developed strategy ended up failing in more complex and sterically
demanding systems.
|
|
| 49
|
Song Z., Samanta R., Antonchick A.P. Rhodium(III)-Catalyzed Direct Regioselective Synthesis of 7-Substituted Indoles. Org. Lett. 2013, 15, 5662-5665, DOI:10.1021/ol402626t
| 
An efficient, atom-economic one-pot method was developed for the preparation of 7-substituted indoles via rhodium(III)-catalyzed oxidative cross-coupling. Regioselective olefination of indoline derivatives followed by one-pot subsequent oxidation provided the desired products in good to excellent yields.
|
|
| 48
|
Narayan R., Bauer J. O., Strohmann C., Antonchick A.P.*, Waldmann H.* Catalytic Enantioselective Synthesis of Functionalized Tropanes Reveals Novel Inhibitors of Hedgehog Signaling. Angew. Chem., Int. Ed. 2013, 52, 12892-12896, DOI:10.1002/anie.201307392. Angew. Chem. 2013, 125, 13130-13134, DOI:10.1002/ange.201307392
Selected as Hot Paper. Highlighted in Synfacts 2014, 272 and ChemInform Editor’s Choice (RxnFinder)
| 
Dipolar cycloaddition: A highly efficient copper(I)-catalyzed enantioselective [3+2] cycloaddition reaction of 1,3-fused cyclic azomethine ylides and nitroalkenes has been developed. This method provides access to functionalized tropane scaffolds with several quaternary and tertiary stereocenters in a single step under mild reaction conditions.
|
|
| 47
|
Takayama H., Jia Z.-J., Kremer L., Bauer J.O., Strohmann C., Ziegler S., Antonchick A.P.*, Waldmann H.* Discovery of Inhibitors of the Wnt and Hedgehog Signaling Pathways through the Catalytic Enantioselective Synthesis of an Iridoid-Inspired Compound Collection. Angew. Chem., Int. Ed. 2013, 52, 12404-12408, DOI:10.1002/anie.201306948. Angew. Chem. 2013, 125, 12630-12634, DOI:10.1002/ange.201306948
| 
Cousins you can count on: An iridoid-inspired compound collection was synthesized efficiently by the resolution of cyclic enones in an asymmetric cycloaddition with azomethine ylides. The collection contained novel potent inhibitors of the Wnt and Hedgehog signaling pathways.
|
|
| 46
|
Potowski M., Antonchick A.P.*, Waldmann H.* Catalytic Asymmetric exo-Selective [6+3] Cycloaddition of Iminoesters with Fulvenes. Chem. Commun. 2013, 49, 7800-7802, DOI:10.1039/C3CC43824D.
| 
A novel exo-selective [6+3] cycloaddition approach for the highly enantioselective synthesis of polysubstituted piperidines was developed. The developed methodology was applied in an one-pot [6+3]/[4+2] dicycloaddition allowing the construction of polycyclic structurally and stereochemically rich compounds from simple building blocks.
|
|
| 45
|
Matcha K., Narayan R., Antonchick A.P., Metal-Free Radical Azidoarylation of Alkenes: Rapid Access to Oxindoles by Cascade C-N and C-C Bond-Forming Reactions. Angew. Chem., Int. Ed. 2013, 52, 7985-7989, DOI:10.1002/anie.201303550. Angew. Chem. 2013, 125, 8143-8147, DOI:10.1002/ange.201303550
| 
A novel method for the oxidative radical azidation of alkenes relies on an azide in combination with a hypervalent iodine reagent. A cascade of C-N and C-C bond-forming reactions yields 2-oxindoles under metal-free conditions with high reaction rates at ambient temperature and provides access to complex products (see scheme; TMS=trimethylsilyl).
|
|
| 44
|
Samanta R., Matcha K., Antonchick A.P. Metal-Free Oxidative Carbon-Heteroatom Bond Formation Through C–H Bond Functionalization. (Invited Minireview) Eur. J. Org. Chem., 2013, 5769-5804, DOI:10.1002/ejoc.201300286
One of the most accessed article in 2013-2015 and the most frequently cited among the articles published in 2013.
| 
The development of new bond formation methods through coupling of simple compounds by direct functionalization of non-reactive C–H bonds is an important area of modern organic synthesis. The current status in the development of synthetic methodologies for carbon-heteroatom bond-forming reactions under metal-free conditions is highlighted.
|
|
| 43
|
Antonchick A.P, López-Tosco S., Parga J., Sievers S., Schürmann M., Preut H., Höing S., Schöler H.R., Sterneckert J., Rauh D., Waldmann H. Highly Enantioselective Catalytic Synthesis of Neurite Growth-Promoting Secoyohimbanes. Chemistry & Biology, 2013, 20, 500-509, DOI:10.1016/j.chembiol.2013.03.011
| 
Natural products endowed with neuromodulatory activity and their underlying structural scaffolds may inspire the synthesis of novel neurotrophic compound classes. The spirocyclic secoyohimbane alkaloid rhynchophylline is the major component of the extracts of Uncaria species used in Chinese traditional medicine for treatment of disorders of the central nervous system. Based on the structure of rhynchophylline, a highly enantioselective and efficient organocatalyzed
synthesis method was developed that gives access to the tetracyclic secoyohimbane scaffold, embodying a quaternary and three tertiary stereogenic centers in a one-pot multistep reaction sequence. Investigation of a collection of the secoyohimbanes in primary rat hippocampal neurons and embryonal stem cell-derived motor neurons led to
discovery of compounds that promote neurite outgrowth and influence the
complexity of neuronal network formation.
|
|
| 42 |
Antonchick A.P., Burgmann L. Direct Selective Oxidative Cross-Coupling of Simple Alkanes with Heteroarenes. Angew. Chem., Int. Ed. 2013, 52, 3267-3271, DOI:10.1002/anie.201209584. Angew. Chem. 2013, 125, 3349-3353, DOI:10.1002/ange.201209584
One of the most accessed articles in February 2013. Highlighted in "Some Items of Interest to Process R&D Chemists and Engineers" Org. Process Res. Dev. 2013, 17 , 724-735 DOI:10.1021/op400094t
|

A dream reaction: An efficient and practical method for the oxidative cross-coupling of heteroaromatic compounds and simple alkanes has been developed. The desired products are smoothly and regioselectively formed under mild reaction conditions at ambient temperature in a hypervalent-iodine-mediated transformation. The method allows for preferential transformation of stronger C(sp3)-H bonds in the presence
of weaker C(sp3)-H bonds under metal-free conditions.
|
|
| 41 |
Matcha K., Antonchick A.P. Metal-Free Cross-Dehydrogenative Coupling of Heterocycles with Aldehydes. Angew. Chem., Int. Ed. 2013, 52, 2082-2086, DOI:10.1002/anie.201208851. Angew. Chem. 2013, 125, 2136-2140, DOI:10.1002/ange.201208851
|

A range of heterocyclic compounds were synthesized by a novel, metal-free cross-dehydrogenative coupling between heterocycles and aldehydes under mild reaction conditions that are not sensitive to moisture. The products are formed smoothly and regioselectively at room temperature by a hypervalent iodine mediated transformation. This method has a broad substrate scope and was used in the highly efficient, one-step synthesis of natural products.
|
|
| 40 |
Samanta R., Narayan R., Antonchick A.P. Rhodium(III)-Catalyzed Direct Oxidative Cross Coupling at the C5 Position of Chromones with Alkenes. Org. Lett. 2012, 14, 6108-6111, DOI:10.1021/ol303067f
|

|
|
An atom-economical, Rh(III)-catalyzed, direct oxidative cross-coupling
at the C5 position of chromones and flavones with a broad range of
alkenes was developed. The products were formed in a highly
regioselective manner in good to excellent yields. The developed method
was applied in the cross-coupling of natural products for the synthesis
of hybrid molecules.
|
|
| 39 |
Samanta R., Bauer J.O., Strohmann C., Antonchick A.P. Organocatalytic, Oxidative, Intermolecular Amination and Hydrazination of Simple Arenes at Ambient Temperature. Org. Lett. 2012, 14, 5518-5521, DOI:10.1021/ol302607y
One of the most read articles in 10/2012–11/2012 and one of the most accessed articles in first year after publication.
|

|
|
New atom-economical, environmental friendly, direct oxidative intermolecular processes of amination and hydrazination of nonprefunctionalized arenes were developed. The products were formed in a good regioselective manner under organocatalytic conditions at ambient temperature.
|
|
| 38 |
Sos M.L., Dietlein F., Peifer M., Schöttle J., Balke-Want H., Müller C., Koker M., Richters A., Heynck S., Malchers F., Heuckmann J.M., Seidel D., Eyers P.A., Ullrich R.T., Antonchick A.P., Vintonyak V.V., Schneider P.M., Ninomiya T., Waldmann H., Büttner R., Rauh D., Heukamk L.C., Thomas R.K. A Framework for Identification of Actionable Cancer Genome Dependencies in Small Cell Lung Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 17034-17039,
DOI:10.1073/pnas.1207310109
|
Small cell lung cancer (SCLC) accounts for about 15% of all lung cancers. The prognosis of SCLC patients is devastating and no biologically targeted therapeutics are active in this tumor type. To develop a framework for development of specific SCLC-targeted drugs we conducted a combined genomic and pharmacological vulnerability screen in SCLC cell lines. We show that SCLC cell lines capture the genomic landscape of primary SCLC tumors and provide genetic predictors
for activity of clinically relevant inhibitors by screening 267 compounds across 44 of these cell lines. We show Aurora kinase inhibitors are effective in SCLC cell lines bearing MYC amplification, which occur in 3–7% of SCLC patients. In MYC-amplified SCLC cells Aurora kinase inhibition associates with G2/M-arrest, inactivation of PI3-kinase (PI3K) signaling, and induction of apoptosis. Aurora dependency in SCLC primarily involved Aurora B, required its kinase activity, and was independent of depletion of cytoplasmic
levels of MYC. Our study suggests that a fraction of SCLC patients may benefit from therapeutic inhibition of Aurora B. Thus, thorough chemical and genomic exploration of SCLC cell lines may provide starting points for further development of rational targeted therapeutic intervention in this deadly tumor type.
|
|
| 37 |
Potowski M., Bauer J.O., Strohmann C., Antonchick A.P*, Waldmann H.* Highly Enantioselective Catalytic [6+3] Cycloadditions of Azomethine Ylides. Angew. Chem., Int. Ed. 2012, 51, 9512-9516, DOI:10.1002/anie.201204394. Angew. Chem. 2012, 124, 9650-9654, DOI:10.1002/ange.201204394
frontispiece
Highlighted in Synfacts 2012, 1350
|

|
|
Under control: Highly functionalized chiral annulated piperidines with eight stereocenters are efficiently obtained by means of a highly enantioselective onepot [6+3]/[4+2] sequence. This sequence included the first enantioselective [6+3] cycloaddition of azomethine ylides with fulvenes.
|
|
| 36 |
Samanta R., Kulikov K., Strohmann C., Antonchick A.P. Metal-Free Electrocyclization at Ambient Temperature: Synthesis of 1-Arylcarbazoles. (invited feature article) Synthesis 2012, 44, 2325-2332, DOI: 10.1055/s-0032-1316743
One of the 10 most accessed articles in 8/2012.
|

|
|
An efficient novel protocol for the construction of an hyellazole-inspired compound collection is described. Starting from 2,6-diarylacetanilides, the desired products were obtained using hypervalent iodine promoted electrocyclization. The mechanism of product formation was investigated through intramolecular competition experiments.
|
|
| 35 |
Potowski M., Schürmann M., Preut H, Antonchick A.P.*, Waldmann H.* Programmable Enantioselective One-pot Synthesis of Molecules With Eight Stereocenters. Nat. Chem. Biol. 2012, 8, 428-430, DOI:10.1038/nchembio.901
|

|
|
We developed an enantioselectively catalyzed tandem synthesis of structurally and stereochemically complex molecules that forms four carbon-carbon bonds and sets eight stereocenters with high regio-, diastereo- and enantioselectivity. It can be programmed to yield different stereoisomers by varying only the order of combination of a common set of reagents and catalysts. We report what is to our knowledge the first synthesis of both enantiomers
of a chiral compound using the same chiral catalyst
|
|
| 34 |
Samanta R., Lategahn J., Antonchick A.P. Metal-free Direct Oxidative Intermolecular Diarylation of Anilides at Ambient Temperature Assisted by Cascade Selective Formation of C–C and C–N bonds. Chem. Commun. 2012, 48, 3194-3196, DOI:10.1039/C2CC30324H
|

|
|
A new atom-economical process of direct oxidative intermolecular functionalization of aniline derivatives by simple arenes was developed. The products were formed in a highly regioselective manner under metal-free conditions at ambient temperature
|
|
| 33 |
Samanta R., Antonchick A.P. Metal-Free Oxidative C-H Bond Amination at Ambient Temperature. (invited highlight) Synlett 2012, 809-813, DOI:10.1055/s-0031-1290531
|

|
|
Direct oxidative methods of C-H bond functionalization represent efficient straightforward approaches in formation of new bonds. Those transformations allow coupling of nonprefunctionalized building blocks and engaged growing attention from academic and industrial communities. Recent results on development of environmentally benign oxidative aminations of C-H bonds are highlighted.
|
|
|
32
|
Antonchick A.P., Samanta R., Kulikov K., Lategahn J. Organocatalytic, Oxidative, Intramolecular C–H Bond Amination and Metal-free Cross-Amination of Unactivated Arenes at Ambient Temperature. Angew. Chem., Int. Ed. 2011, 50, 8605-8608, DOI:10.1002/anie.201102984. Angew. Chem. 2011, 123, 8764-8767, DOI:10.1002/ange.201102984
Selected as Hot Paper. One of the most accessed articles in August 2011 and one of the most accessed articles in first year after publication. Highlighted in Synfacts 2011, 1129.
|

The twinkling of an I: In a new atom-economical and environmentally friendly organocatalytic method for intramolecular C-H amination, the C-N bond forms at ambient temperature by abstraction of two atoms of hydrogen; only acetic acid and water are formed as by-products. The method has also been extended to the unprecedented metal-free cross-amination of nonactivated arenes.
|
|
| 31 |
Stöckigt J., Antonchick A.P., Wu F., Waldmann H. The Pictet-Spengler Reaction in Nature and in Organic Chemistry. Angew. Chem., Int. Ed. 2011, 50, 8538-8564, DOI:10.1002/anie.201008071. Angew. Chem. 2011, 123, 8692-8719, DOI:10.1002/ange.201008071
One of the most accessed articles in August 2011 and one of the most accessed articles in first year after publication.
| 
|
Alkaloids are an important class of natural products that are widely distributed in nature and produced by a large variety of organisms. They have a wide spectrum of biological activity and for many years were used in folk medicine. These days, alkaloids also have numerous applications in medicine as therapeutic agents. The importance of these natural products in inspiring drug discovery programs is proven
and, therefore, their continued synthesis
is of significant interest. The condensation discovered by Pictet and Spengler is the most important method for the synthesis of alkaloid scaffolds. The power of this synthesis method has been convincingly proven in the construction of stereochemicaly and structurally complex alkaloids.
|
|
| 30 |
Samanta R., Antonchick A.P. Palladium-Catalyzed Double C–H Activation Directed by Sulfoxides in the Synthesis of Dibenzothiophenes. Angew. Chem., Int. Ed. 2011, 50, 5217-5220, DOI:10.1002/anie.201100775. Angew. Chem. 2011, 123, 5323-5326, DOI:10.1002/ange.201100775
Highlighted in Synfacts 2011, 836.
|

|
|
S=O shows where to go: A novel double C-H activation of aromatic compounds with a sulfoxide as a directing group results in the highly regioselective synthesis of polysubstituted dibenzothiophenes (see scheme). The reaction cascade consists of palladium-catalyzed double C-H activation and a Pummerer rearrangement followed by palladium-catalyzed C-S bond formation.
|
|
| 29 |
Schuster H., Martinez R., Bruss H., Antonchick A.P., Kaiser M., Schürmann M., Waldmann H. Synthesis of the B-seco limonoid scaffold. Chem. Commun. 2011, 47, 6545-6547, DOI:10.1039/C1CC11388G
|

The underlying stereochemically complex and densely functionalized scaffold of the B-seco limonoids was synthesized employing an Ireland–Claisen rearrangement as key transformation.
|
|
| 28 |
Antonchick A.P., Schuster H., Bruss H., Schurmann M., Preut H., Rauh D., Waldmann H. Enantioselective synthesis of the spirotryprostatin A scaffold. (invited manuscript in honor of Gilbert J. Stork) Tetrahedron 2011, 67, 10195-10202 DOI:10.1016/j.tet.2011.04.056
|

|
|
The pentacyclic spirotryprostatin scaffold embodies an oxindole with an all-carbon quaternary stereocenter. The scaffold can efficiently be accessed in a one-pot reaction sequence consisting of a highly enantioselective 1,3-dipolar cycloaddition, N-acylation of the resulting stereochemically complex 3,3′-pyrrolidinyl-spirooxindole core with Fmoc-proline and spontaneous ring closure upon N-deprotection.
|
|
| 27 |
Rueping M., Theissmann T., Stoeckel M., Antonchick A.P. Direct enantioselective access to 4-substituted tetrahydroquinolines by catalytic asymmetric transfer hydrogenation of quinolines. Org. Biomol. Chem. 2011, 9, 6844-6850 DOI: 10.1039/C1OB05870C
|

|
|
A convenient protocol for the enantioselective synthesis of 4-substituted tetrahydroquinolines has been developed. Chiral BINOL phosphoric acids promote the reduction of a wide range of 4-substituted quinolines with Hantzsch esters with good to high levels of enantioselectivity.
|
|
| 26 |
Antonchick A.P., Gerding-Reimers C., Catarinella M., Schürmann M., Preut H., Ziegler S., Rauh D., Waldmann H. Highly Enantioselective Synthesis and Cellular Evaluation of Natural Product-Inspired Spirooxindoles. Nat. Chem. 2010, 2, 735-740. DOI:10.1038/nchem.730
Highlighted in Synfacts 2010, 1379.
|

|
|
In biology-oriented synthesis the underlying scaffold classes of natural products selected in evolution are used to define biologically relevant starting points in chemical structure space for the synthesis of compound collections with focused structural diversity. Here we describe a highly enantioselective synthesis of natural-product-inspired 3,3'-pyrrolidinyl spirooxindoles - which contain an all-carbon quaternary centre and three tertiary stereocentres.
This synthesis takes place by means of an asymmetric Lewis acid-catalysed 1,3-dipolar cycloaddition of an azomethine ylide to a substituted 3-methylene-2-oxindole using 1–3 mol% of a chiral catalyst formed from a N,P-ferrocenyl ligand and CuPF6(CH3CN)4. Cellular evaluation has identified a molecule that arrests mitosis, induces multiple microtubule organizing centres and multipolar
spindles, causes chromosome congression defects during mitosis and inhibits tubulin regrowth in cells. Our findings support the concept that compound collections based on natural-product-inspired scaffolds constructed with complex stereochemistry will be a rich source of compounds with diverse bioactivity.
|
|
| 25
|
Rueping M., Brinkmann C., Antonchick A.P., Atodiresei I. Asymmetric Synthesis of Indolines by Catalytic Enantioselective Reduction of 3H-Indoles. Org. Lett. 2010, 12, 4604-4607. DOI:10.1021/ol1019234
|

|
|
A highly enantioselective metal-free reduction of 3H-indoles has been developed. This Brønsted acid catalyzed transfer hydrogenation of indole derivatives with Hantzsch dihydropyridine as the hydrogen source constitutes an efficient method for the synthesis of various optically active indolines with high enantioselectivities.
|
|
| 24 |
Vintonyak V.V., Antonchick A.P., Rauh D., Waldmann H. The Therapeutic Potential of Phosphatase Inhibitors. Curr. Opin. Chem. Biol. 2009, 13, 272-283. DOI:10.1016/j.cbpa.2009.03.021
|
Protein phosphatases (PPs) constitute a large family of enzymes, which are
crucial modulators of cellular phosphorylation events. Malfunction in PP
activity has been associated with human diseases, including diabetes, obesity,
cancer, and neurodegenerative and autoimmune disorders, and makes this class of
enzymes attractive targets for chemical biology and medicinal chemistry
research. A number of strategies are currently explored for the identification
and development of various classes of PP modulators and have resulted in a
plethora of chemically distinct inhibitors. Limited selectivity and adverse
pharmacological properties of PP inhibitors are still major bottlenecks for
further clinical development and resulted in only a few molecular entities
currently in clinical trials
|
|
| 23 |
Rueping M., Antonchick A.P., Sugiono E., Grenader K. Asymmetric Brønsted Acid Catalysis: Catalytic Enantioselective Synthesis of Highly Biologically Active Dihydroquinazolinones. Angew. Chem., Int. Ed. 2009, 48, 908-910, DOI:10.1002/anie.200804770. Angew. Chem.
2009, 121, 925-927, DOI:10.1002/ange.200804770
|

|
|
Surprisingly straightforward: A metal-free, highly enantioselective Brønsted acid catalyzed condensation/addition reaction has been developed for the construction of 2,3-dihydroquinazolinones starting from 2-aminobenzamide and aldehydes (see scheme). This efficient approach provides 2,3-dihydroquinazolinones with a strong preference for the S-enantiomers, which have higher biological activities than the R-enantiomers.
|
|
| 22 |
Rueping M., Antonchick A.P. Catalytic Asymmetric Aminoallylation of Aldehydes: A Catalytic Enantioselective Aza-Cope Rearrangement. Angew. Chem., Int. Ed. 2008, 47, 10090-10093, DOI:10.1002/anie.200803610. Angew.Chem. 2008, 120, 10244-10247, DOI:10.1002/ange.200803610
Highlighted in Synfacts 2009, 210. One of the most accessed articles in November 2008
|

|
|
A condensation–rearrangement sequence forms the basis of a high-yielding route to chiral homoallylic amines from readily accessible aldehydes (see scheme). This transformation is both the first enantioselective Brønsted acid catalyzed sigmatropic rearrangement and the first example of a catalytic asymmetric aza-Cope rearrangement.
|
|
| 21 |
Rueping M., Antonchick A.P. A Highly Enantioselective Brønsted Acid Catalyzed Reaction Cascade. Angew. Chem., Int. Ed. 2008, 47, 5836 - 5838, DOI:10.1002/anie.200801435. Angew. Chem. 2008, 120, 5920-5922, DOI:10.1002/ange.200801435
Highlighted in Synfacts 2008, 994.
|

|
|
One cat. is enough! A highly enantioselective reaction has been developed for the three-component reaction of an enamine with a vinyl ketone and a Hantszsch ester in which each of the six reaction steps is catalyzed by the same chiral Brønsted acid (see scheme). This reaction offers efficient access to tetrahydropyridines and azadecalinones from simple and readily available starting materials.
|
|
| 20
|
Rueping M., Antonchick A.P. Brønsted-Acid-Catalyzed Activation of Nitroalkanes: A Direct Enantioselective Aza-Henry Reaction. Org. Lett. 2008, 10, 1731-1734, DOI:10.1021/ol8003589
|

|
|
A direct asymmetric organocatalytic aza-Henry reaction has been developed in which a new bifuctional Brønsted-acid-catalyzed activation of nitroalkanes provides an efficient access to α,β-diamino acids with high dia- and enantioselectivities under mild and base-free reaction conditions.
|
|
| 19 |
Rueping M., Antonchick A.P., Brinkmann C. Dual Catalysis: A Combined Enantioselective Bronsted Acid and Metal-Catalyzed Reaction - Metal Catalysis with Chiral Counterions. Angew. Chem., Int. Ed. 2007, 46, 6903-6906, DOI:10.1002/anie.200702439. Angew. Chem. 2007, 119, 7027-7030, DOI:10.1002/ange.200702439
|

|
|
Double take: The combination of enantioselective Brønsted acid catalyzed activation and metal-catalyzed alkynylation of α-imino esters under mild reaction conditions leads to amino acids in high yields and with excellent e.r. values (see scheme; PG=protecting group).
|
|
| 18 |
Rueping M., Antonchick A.P. Organocatalytic Enantioselective Reduction of Pyridines. Angew. Chem., Int. Ed. 2007, 46, 4562 - 4565, DOI:10.1002/anie.200701158. Angew. Chem. 2007 , 119, 4646-4649, DOI:10.1002/ange.200701158
One of the most accessed articles in May 2007. Highlighted in Synfacts 2007, 912, and Organic Process Research & Development 2007, 11, 787 "Highlights from the literature".
|

|
|
Metal-free at last! The first enantioselective organocatalytic reduction of pyridine derivatives leads to hexahydrochinolinones and tetrahydropyridines in good yields and with excellent enantioselctivities (up to 92% ee; see scheme). These compounds are starting materials for the synthesis of various natural products.
|
|
| 17 |
Rueping M., Ieawsuwan W., Antonchick A.P., Nachtsheim B. J. Chiral Brønsted Acids in the Catalytic Asymmetric Nazarov Cyclization – The First Enantioselective Organocatalytic Electrocyclic Reaction. Angew. Chem., Int. Ed. 2007, 46, 2097 - 2100, DOI:10.1002/anie.200604809. Angew. Chem. 2007, 119, 2143-2146,
DOI:10.1002/ange.200604809
Selected as Hot Paper. One of the most accessed articles in March 2007. Highlighted in Science 2007, 315, 1055, and in Synfacts 2007, 432.
|

|
|
Low catalyst loadings, high enantioselectivities, mild conditions, and fast reaction times are the important features of the first enantioselective organocatalytic electrocyclic reaction: a Nazarov cyclization leading to the synthesis of substituted five-membered rings with a chiral Brønsted acid as a catalyst. A further advantage of this method is the possible entry to all four diastereomers of the product.
|
|
| 16 |
Rueping M., Antonchick A.P., Theissmann T. Remarkably Low Catalyst Loading in Brønsted Acid Catalyzed Transfer Hydrogenations: Enantioselective Reduction of Benzoxazines, Benzothiazines, and Benzoxazinones. Angew. Chem., Int. Ed. 2006, 45, 6751-6755, DOI:10.1002/anie.200601832 Angew. Chem. 2006,
118, 6903-6907, DOI:10.1002/ange.200601832
|

|
|
A highly efficient transfer hydrogenation of benzoxazines, benzothiazines, and benzoxazinones with as low as 0.01 mol% binol phosphate catalyst furnishes the dihydro-2H-benzoxazines, -benzothiazines, and -benzoxazinones in good yields and with excellent enantioselectivities. Particularly noteworthy are the mild reaction conditions and the wide scope of substrates, including sulfur-containing compounds.
|
|
| 15
|
Rueping M., Antonchick A.P., Theissmann T. A Highly Enantioselective Brønsted Acid Catalyzed Cascade Reaction: Organocatalytic Transfer Hydrogenation of Quinolines and their Application in the Synthesis of Alkaloids. Angew. Chem., Int. Ed., 2006, 45, 3683-3686, DOI:10.1002/anie.200600191. Angew.
Chem. 2006, 118, 3765-3768, DOI:10.1002/ange.200600191
Selected as Hot Paper. Highlighted in Angew. Chem. Int. Ed. 2007, 46, 1570. See also: Organic Process Research & Development 2006, 10, 853. and in Trendberichte in Nachrichten aus der Chemie 2007, 55, 263, as well as in Synfacts 2006,
731.
One of the most
frequently cited articles published in
Angewandte Chemie in 2006 or 2007.
|

|
|
A categorical success: A Brønsted acid catalyzed cascade transfer hydrogenation provides direct access to 2-aryl- and 2-alkyl-substituted tetrahydroquinolines with excellent enantioselectivities under mild conditions and using very low amounts of catalyst (see scheme). The best results were achieved with the binol phosphate catalyst, where Ar=9-phenanthryl, used successfully in the Strecker reaction.
|
|
|
14
|
Rueping M., Theissmann T., Antonchick A.P. Metal-free Brønsted Acid Catalyzed Transfer-Hydrogenation: Enantioselective Synthesis of Tetrahydroquinolines. Catalysts for Fine Chemical Industry, 2007, Vol. 5, Regio- and Stereo-Controlled Oxidations and Reductions. Ed. S.M. Roberts, J. Whittall, ch. 4.2, 170-174. ISBN:
978-0-470-09022-0
|
|
13
|
Rueping M., Theissmann T., Antonchick A.P. Metal-Free Brønsted Acid Catalyzed Transfer Hydrogenation - New Organocatalytic Reduction of Quinolines. Synlett 2006, 1071-1074, DOI: 10.1055/s-2006-939706.
|

|
|
The first metal-free Brønsted acid catalyzed hydrogenation of quinolines using Hantzsch dihydropyridine as the hydrogen source has been developed. This, so far unprecedented organocatalytic reduction of heteroaromatic compounds provides a variety of differently substituted 1,2,3,4-tetrahydroquinolines in excellent yields under mild reaction conditions using a remarkably low amount of Brønsted acid catalyst.
|
|
|
12
|
Khripach V.A., Zhabinskii V.N., Antonchick A.V., and Antonchick A.P. Synthesis of (24S)-Hydroxy and (24S)-24,25-Epoxycholesterol Analogues, Potential Agonists of Nuclear LXR Receptors. Russ. J. Bioorg. Chem. 2006, 32, 586–594. DOI:10.1134/S1068162006060124
|
|
|
A new approach to the synthesis of
a series of isomeric 24-hydroxy-and 24,25-epoxysteroids starting from
lithocholic acid was proposed. Sharpless asymmetric hydroxylation of
intermediate Δ24-olefines was used as a reaction determining the
stereochemistry of target compounds. The resulting derivatives are potential
agonists of nuclear receptors LXRα and LXRβ and are
potentially useful in the structure-function studies.
|
|
|
11
|
Antonchick A.P., Svatos A., Konstantinova O.V., Zhabinskii V.N., Khripach V.A., Schneider B. Reversible Conversion in the Brassinosteroid Quartet Castasterone, Brassinolide and their 3β-Epimers. Z. Naturforsch. 2006, 61b, 1039-1044.
|
|
|
The metabolism of deuterated brassinosteroids has been studied in excised leaves of Secale cereale, and in vitro in seedlings of Arabidopsis thaliana and cell suspension cultures of Lycopersicon esculentum. In addition to the known biosynthetic conversion of castasterone to brassinolide and epimerization to 3-epicastasterone, inversion of the 3a-configured hydroxyl group of brassinolide to a 3α-configured one in 3-epibrassinolide has
been
observed using liquid chromatography (HPLC) with electrospray ionization (ESI) and selected ion-monitoring mass-spectrometry (SIM-MS). Administration of deuterated 3-epicastasterone and 3-epibrassinolide to Arabidopsis and Secale seedlings resulted in the formation of castasterone and brassinolide, respectively, indicating conversion of configuration at C-3 of brassinosteroids is reversible.
|
|
|
10
|
Khripach V.A., Zhabinskii V.N., Konstantinova O.V., Khripach N.B., Antonchick A.V., Antonchick A.P., and Schneider B. Preparation of (25R)- and (25S)-26-Functionalized Steroids as Tools for Biosynthetic Studies of Cholic Acids. Steroids 2005, 70, 551-562, DOI:10.1016/j.steroids.2005.02.014
|
|
A new synthesis of both epimeric forms of 26-cholestanoic acids and
26-alcohols containing a 3β-hydroxy-Δ5- or a Δ4-3-keto-functionality in ring A is described starting from stigmasterol
or (20S)-3β-acetoxy-pregn-5-en-20-carboxylic acid. The obtained compounds are
useful as standards for studies of cholic acids. Construction of the side chain
was achieved by linkage of steroidal 23-iodides to sulfones prepared from (2R)-
and (2S)-3-hydroxy-2-methylpropanoates. Oxidation of intermediate 26-alcohols
into the corresponding carboxylic acids ensuring preservation of stereochemistry
at C-25 and functional groups in the cyclic part was achieved with sodium
chlorite catalyzed by TEMPO and bleach.
|
|
|
9
|
Antonchick A.P., Svatos A., Schneider B., Konstantinova O.V., Zhabinskii V.N., Khripach V.A. 2,3-Epoxybrassinosteroids Are Intermediates in the Biosynthesis of Castasterone in Seedlings of Secale cereale. Phytochemistry 2005, 66, 65-72, DOI:10.1016/j.phytochem.2004.11.008
|

|
|
The involvement of the 2,3-epoxybrassinosteroids secasterone and 2,3-diepisecasterone in the biosynthesis of castasterone has been demonstrated in seedlings of Secale cereale by LC–ESI-MS. Deuterated secasterone, upon administration to rye seedlings, was incorporated into castasterone and its 2β- and 3β-epimers. Administration of deuterated 2,3-diepisecasterone resulted in castasterone and 2-epicastasterone.
A biosynthetic subpathway from typhasterol/teasterone via 2,3-epoxybrassinosteroid intermediates to castasterone is discussed.
|
|
|
8
|
Antonchick A.P., Schneider B., Zhabinskii V.N., Khripach V.A. Synthesis of [26,27-2H6]Brassinosteroids From 23,24-Bisnorcholenic Acid Methyl Ester. Steroids 2004, 69, 617-628, DOI:10.1016/j.steroids.2004.05.014
|
|
|
A number of hexadeuterated brassinosteroids (BS) containing a hydroxy group at C-22 or a 22R,23R-diol function were prepared starting from 23,24-bisnorcholenic acid methyl ester for biosynthetic studies. Synthesis of the cyclic part was accomplished via the initial hydroboration–oxidation of Δ5-double bond. The key step in the synthesis of the side chain involved addition of (2S)-[3,4-2H6]2,3-dimethylbutylphenyl
sulfone to the corresponding C-22 aldehydes
|
|
|
7
|
Svatos A., Antonchick A., Schneider B. Determination of Brassinosteroids in the Sub-femtomolar Range Using Dansyl-3-aminophenylboronate Derivatization and Electrospray Mass Spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 816-821, DOI:10.1002/rcm.1413
|
|
|
A selective and sensitive electrospray ionization (ESI) mass spectrometry based method for detection of brassinosteroids (BS) in plant samples was developed. The limit of detection (LOD) was dramatically reduced over existing analytical methods using a microbore (1.00 mm) C18 column and chemical derivatization of free BS to dansyl-3-aminophenylboronates. The LOD in the selected-ion monitoring (SIM) mode for derivatized BS was 125 attomole (signal-to-noise
ratio 3). The practical utility of the method is documented in Arabidopsis thaliana plant transformation of castasterone to brassinolide using a deuterium-labeled precursor. The method could be very useful for the detection of native BS in plant tissue and biosynthetic studies.
|
|
|
6
|
Antonchick A.P., Schneider B., Zhabinskii V.N., Konstantinova O.V., Khripach V.A. Biosynthesis of 2,3-Epoxybrassinosteroids in Seedlings of Secale cereale. Phytochemistry 2003, 63, 771-776, DOI:10.1016/S0031-9422(03)00354-6
|

|
|
Two new brassinosteroids, (22R,23R,24S)-22,23-dihydroxy-24-methyl-5α-cholest- 2-en-6-one (secasterol) and (22R,23R,24S)-22,23-dihydroxy-2α,3α-epoxy-24- methyl-5α-cholest-6-one (2,3-diepisecasterone) have been identified together with a known 2,3-epoxybrassinosteroid, secasterone, in seedlings of Secale cereale. Deuterated secasterol, teasterone, and typhasterol, upon
administration to rye seedlings, were incorporated into secasterone and 2,3-diepisecasterone, indicating a biosynthetic route via teasterone/typhasterol to secasterol to 2,3-epoxybrassinosteroids.
|
|
|
5
|
Khripach V.A., Zhabinskii V.N., Antonchick A.P., Konstantinova O.V., Schneider B. Synthesis of Hexadeuterated 23-Dehydroxybrassinosteroids. Steroids 2002, 67, 1101-1108, DOI:10.1016/S0039-128X(02)00071-5
|
|
|
Two hexadeuterated brassinosteroids (BS) ([26,27-2H6]-23-dehydroxycastasterone and [26,27-2H6]-cathasterone) containing a hydroxy group at C22 instead of the 22R,23R-diol function characteristic for most compounds of this class were prepared for biochemical studies. The corresponding non-deuterated compounds are considered intermediates in brassinolide
biosynthesis. The carbon skeleton
of the side chain with proper stereochemistry at C24 was prepared from commercially available (2R)-3-hydroxy-2-methylpropanoate. This low molecular fragment was coupled to the tetracyclic steroidal fragment through the reaction of the appropriate sulfone with C22 aldehyde. Formation of the necessary configuration of the 22-hydroxy group was achieved by hydride reduction of the corresponding ketone. Deuterium atoms at C26 and C27 originated from [2H3]methyl
iodide used for alkylation of the intermediate sulfone.
|
|
|
4
|
Khripach V.A., Zhabinskii V.N., Konstantinova O.V., Antonchick A.P., Schneider B. Synthesis of [26-2H3]Brassinosteroids. Steroids 2002, 67, 587-595, DOI:10.1016/S0039-128X(02)00004-1
|
|
|
A number of [26-2H3]brassinosteroids were prepared for biochemical studies. The parent, nondeuterated compounds were considered to be biosynthetic intermediates in brassinosteroid biosynthesis. Claisen rearrangement was used to construct the steroidal side chain. Deuterium was introduced by reducing the corresponding intermediates with lithium aluminium deuteride.
|
|
|
3
|
Khripach V.A., Zhabinskii V.N., Konstantinova, O.V. Khripach, N.B., Antonchick A.P. Synthesis of 24-Functionalized Oxysterols. Russ. J. Bioorg. Chem. 2002, 28, 257-261, DOI:10.1023/A:1015720707433
|
|
|
The syntheses of (24S)-24,25-epoxycholesterol, (24S)-hydroxycholesterol, and 24-ketocholesterol are described. The compounds belong to oxysterols, which can be considered to be the modulators of cholesterol metabolism. The asymmetric hydroxylation of desmosterol acetate according to Sharpless was used as the key reaction in the stereoselective introduction of functionality in position 24.
|
|
|
2
|
Khripach V.A., Zhabinskii V.N., Konstantinova O.V., Khripach N.B., Antonchick A.P., Schneider, B. [3,3]-Claisen Rearrangements in 24α-Methyl Steroids Synthesis. Application to Campesterol, Crinosterol and Δ25-Crinosterol Side Chain Construction. Steroids 2002, 67, 597-603, DOI:10.1016/S0039-128X(02)00007-7
|
|
|
This paper elaborates an improved synthesis of crinosterol and campesterol starting from stigmasterol. The proposed approach is based on Claisen rearrangement of Δ23-22-allylic alcohols with various configurations of the 22-hydroxy group and geometry of the Δ23-double bond. It allows complete use of the starting steroid for preparing 24α-methyl derivatives. It was possibe to partially control the stereochemistry at C-25. Hydrogenation of the
Δ22-double bond was shown to proceed with partial isomerization of the C-24 alkyl substituent. The Ireland ester enolate variant of the Claisen rearrangement was demonstrated to be useful for preparing 24α-methyl steroids containing the Δ22,25-system.
|
|
|
1
|
Konstantinova O.V., Antonchick A.P., Oldham N.J., Zhabinskii V.N., Khripach V.A., Schneider B. Analysis of Underivatized Brassinosteroids by HPLC/ACPI-MS. Occurrence of 3-Epibrassinolide in Arabidopsis thaliana. Collect. Czech. Chem. Commun. 2001, 66, 1729-1734, DOI:10.1135/cccc20011729
|

|
|
In the course of investigations on brassinosteroid (BS) biosynthetic and metabolic pathways in plants, an approach using HPLC/APCI-MS analysis of underivatized BS for their identification in plant material has been developed. Its application to root-callus suspension cultures of Arabidopsis thaliana led to the first identification of 3-epibrassinolide as natural brassinosteroid.
|
|